10 Small Submissions
No more than 10 Documents per submission
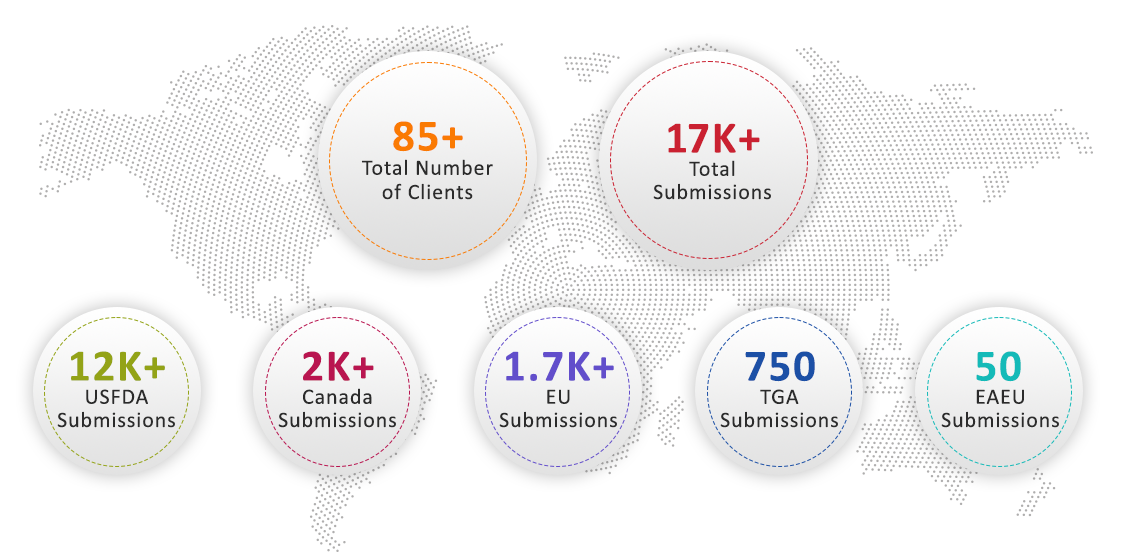
Across the Globe and All Regional eCTD Formats
Freyr SUBMIT PRO - a proven eCTD software for the life sciences industries.
Pharmaceutical | Biotechnology
Freyr SUBMIT PRO, the best eCTD software, supports diverse range of submission templates and formats required by the health authorities world-wide
INDs | NDAs | ANDAs | MAA | NDS
ANDS | DMF | ASMF | IMPD | BLAs
Freyr SUBMIT PRO, a cloud-based eCTD software, aligns with all major health authority regulations
US FDA | EMA | HEALTH CANADA | SWISSMEDIC
SFDA | SAHPRA/MCCZA | TGA | EAEU | JFDA | Thai FDA | ASEAN | NMPA
Commitment l Technology l ROI
Freyr SUBMIT PRO, a comprehensive eCTD Software helps you lower the costs associated with each eCTD submission as it is designed keeping in mind the process improvements such as cloning, parallel submissions, etc.

It is all at one place, Freyr SUBMIT PRO - a comprehensive eCTD submission software makes the entire process hassle-free as it includes an inbuilt eCTD validator and PDF manager, submissions tracker, and HA query management tool.
With comprehensive and custom-built unique features, Freyr SUBMIT PRO acts as a one-stop eCTD solution for all your eCTD submissions.

The inbuilt eCTD validator in our eCTD publishing software supports all the regional and ICH validation criteria and can identify as many as 800+ error scenarios.
Reporting system that can track the number of eCTD submissions, deadlines, and helps you decide on priority submissions.
Manage and track all the Health Authority queries at one place, thereby enabling speedy approvals.
With our very own eCTD submissions solution suite, Freyr SUBMIT PRO seamlessly integrates with rDMS that helps in centralization and easy access of data.
Enabling Organizations Effortlessly Address their Submission Requirements